
Instead, practicing oncologists treat a broader, more heterogeneous patient population under clinical conditions that are often time- and resource-limited. Patient-reported outcomes data demonstrated improved global health status/quality of life at 21 weeks with pembrolizumab-chemotherapy compared with placebo-chemotherapy in KEYNOTE-189 8.Ĭlinical trials, such as KEYNOTE-189, are designed to maximize internal validity by enrolling patients with adequate organ function, good performance status, and no select comorbidities, who then receive therapy in settings with predetermined care. This regimen is recommended for patients with good performance status irrespective of tumor PD-L1 tumor proportion score (TPS), except in the presence of an oncogene predicting lack of benefit or other contraindication to PD-1/PD-L1 inhibitor therapy. National Comprehensive Cancer Network (NCCN) guidelines currently list the combination of pembrolizumab, pemetrexed, and carboplatin or cisplatin as the category 1 preferred regimen for advanced/metastatic adenocarcinoma, large cell, and NSCLC not otherwise specified (NOS) in the first-line setting 1.
#KEYNOTE 189 OVERALL SURVIVAL UPDATE PLUS#
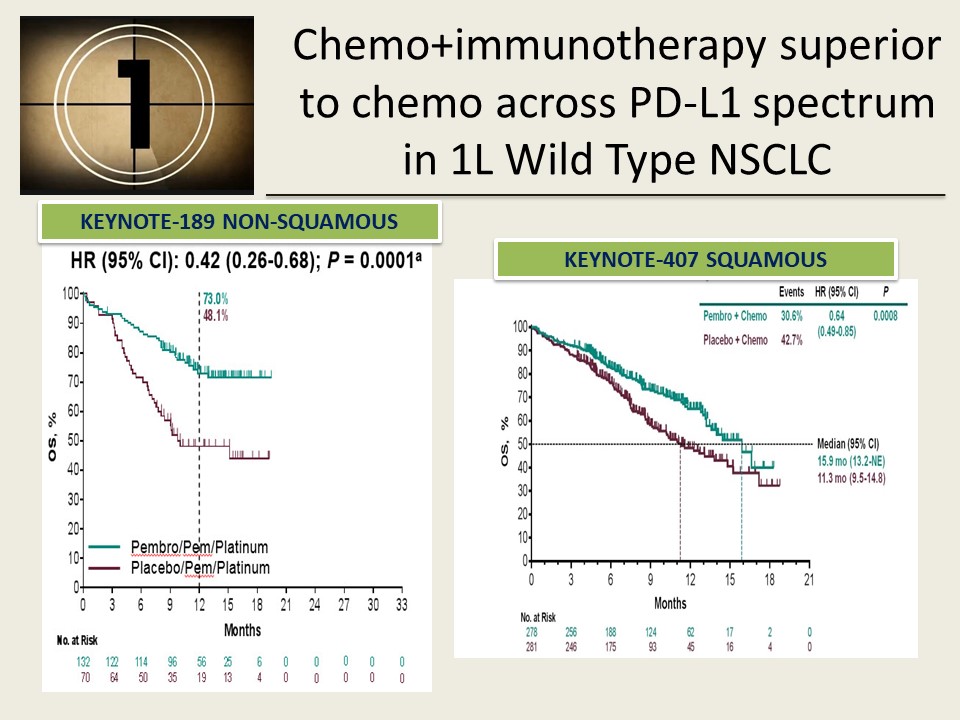
Furthermore, a recently published pooled analysis of three clinical trials found clinically meaningful response and survival benefits and manageable safety for patients with PD-L1-negative advanced NSCLC, both nonsquamous and squamous, who received pembrolizumab plus chemotherapy versus chemotherapy alone 7. Survival benefits were recorded across PD ligand 1 (PD-L1) tumor expression categories 4, 5, 6. Patients who received pembrolizumab plus pemetrexed-platinum experienced significantly longer overall survival (OS) and progression-free survival (PFS) as compared with those who received chemotherapy alone 4, 5. These results were further supported by subsequent findings of the randomized, placebo-controlled, double-blind KEYNOTE-189 trial, which indicated significant benefits of adding first-line pembrolizumab to standard chemotherapy of pemetrexed and a platinum-based drug for patients with metastatic nonsquamous NSCLC without epidermal growth factor receptor ( EGFR) or anaplastic lymphoma kinase ( ALK) mutations. In the United States (US), the results of the phase 2 KEYNOTE-021G study 3 led to accelerated approval on May 10, 2017, by the US Food and Drug Administration (FDA) of first-line pembrolizumab plus chemotherapy for advanced nonsquamous NSCLC. The availability of immune checkpoint inhibitors of the programmed death 1 (PD-1) pathway, including pembrolizumab, nivolumab, atezolizumab, and durvalumab, has recently transformed the choices of systemic anticancer therapy for late-stage non-small cell lung cancer (NSCLC). Lung cancer is often diagnosed at advanced stages when surgical removal is not feasible therefore, the choice of first-line systemic therapy is important 1, 2. These findings demonstrate the effectiveness of pembrolizumab plus pemetrexed-carboplatin by describing clinical outcomes among patients with metastatic nonsquamous NSCLC who were treated at US oncology practices. At data cutoff on August 31, 2019, median patient follow-up was 20.3 months (range 12–28 months), and median real-world times on treatment (rwToT) with pembrolizumab and pemetrexed were 5.6 (95% CI 4.5–6.4) and 2.8 months (95% CI 2.2–3.5), respectively.
Of 283 eligible patients, 168 (59%) were male median age was 66 years (range 33–84) and the proportions of patients with PD-L1 tumor proportion score (TPS) of ≥ 50%, 1–49%, < 1%, and unknown were 28%, 27%, 28%, and 17%, respectively.
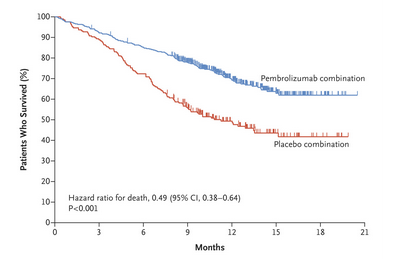
Time-to-event analyses were performed using the Kaplan–Meier method.
#KEYNOTE 189 OVERALL SURVIVAL UPDATE MANUAL#
An enhanced manual chart review was used to collect outcome information. Eligible patients had ECOG performance status of 0–1. Using an anonymized, nationwide electronic health record-derived database, we identified patients who initiated pembrolizumab plus pemetrexed-carboplatin in the first-line setting (May 2017 to August 2018) after diagnosis of metastatic nonsquamous NSCLC that tested negative for EGFR and ALK genomic aberrations. Our aim was to describe outcomes for patients treated with first-line pembrolizumab-combination therapy for metastatic nonsquamous NSCLC in US oncology practices. Evidence from real-world clinical settings is lacking with regard to first-line immunotherapy plus chemotherapy for the treatment of non-small cell lung cancer (NSCLC).


 0 kommentar(er)
0 kommentar(er)
